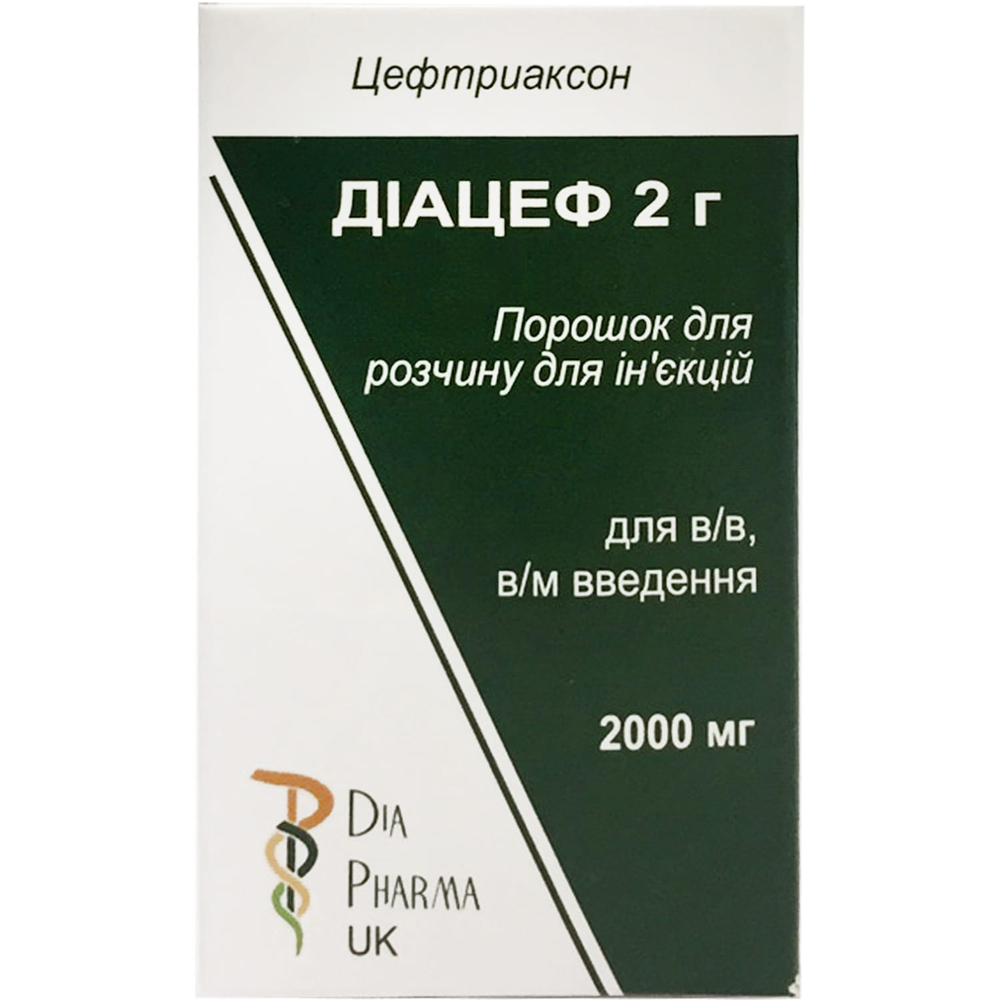
DIACEF 2 G

Jak stosować DIACEF 2 G
Tłumaczenie AI
Ta strona ma charakter informacyjny i nie zastępuje konsultacji lekarskiej. Przed zastosowaniem jakiegokolwiek leku skonsultuj się z lekarzem. Jeśli objawy są poważne, skorzystaj z pilnej pomocy medycznej.
Pokaż oryginałINSTRUCTIONS for medical use of the medicinal product INHAPHYTOL-2
Composition
active substances: 1 g of collection contains chamomile flowers (Matricariae flores) 500 mg, eucalyptus prutovoid leaves (Eucalypti viminalis folia) 500 mg.
Pharmaceutical form
Collection.
Main physical and chemical properties
A mixture of non-uniform particles of light green, gray-green, brown-green, greenish-yellow, yellow, yellowish-brown, yellowish-white color with brown inclusions.
Pharmacotherapeutic group
Means used for cold diseases. ATX code R05X.
Pharmacological properties
Pharmacodynamics
The medicinal product has antimicrobial, anti-inflammatory, reparative, and expectorant effects; exhibits bactericidal and bacteriostatic activity against streptococci, staphylococci, dysentery and intestinal bacilli, against purulent and anaerobic microorganisms.
Clinical characteristics
Indications
Acute and chronic respiratory diseases (pharyngitis, laryngitis, tracheitis, bronchitis).
Contraindications
Increased sensitivity to the components of the medicinal product.
Interaction with other medicinal products and other types of interactions
Unknown.
Features of application
"Inhaphytol-2" is a traditional medicinal product for use in accordance with the indications confirmed by long-term use.
The patient should consult a doctor if the symptoms of the disease do not disappear within one week of using the medicinal product "Inhaphytol-2" or if side reactions not specified in the instructions for medical use of the medicinal product are observed.
Use during pregnancy or breastfeeding
Not recommended due to the lack of special studies.
Ability to affect the speed of reaction when driving vehicles or other mechanisms
Does not affect.
Method of application and doses
The medicinal product is used for inhalations and rinses.
2 tablespoons of the collection should be placed in an enameled dish, poured with 200 ml (1 glass) of hot boiled water, covered with a lid, brought to a boil over low heat, removed from heat, and used for inhalation for 5-10 minutes.
The prepared infusion should be strained, squeezed, and used undiluted in a warm form for rinsing the throat with 1/2 glass 3-4 times a day.
The duration of the treatment course is determined by the doctor individually.
The prepared infusion should be stored in the refrigerator (at a temperature of 2-8 °C) for no more than 2 days.
Children
The medicinal product should be used in children from 6 years old for inhalations; for rinses, it should be used in children from 12 years old as prescribed by a doctor.
Overdose
In case of prolonged and frequent inhalation use, dryness of the mucous membranes of the respiratory organs may occur.
Side reactions
Possible allergic reactions (hyperemia, rash, itching, skin swelling, urticaria, contact dermatitis).
If the symptoms of the disease do not disappear within 1 week of using the medicinal product or if any unwanted side reactions not specified in this instruction appear, the use of the preparation should be stopped and a doctor should be consulted.
Reporting of side reactions after registration of the medicinal product
Is of great importance. This allows monitoring the benefit/risk ratio when using this medicinal product. Medical and pharmaceutical workers, as well as patients or their legal representatives, should report all cases of suspected side reactions and lack of efficacy of the medicinal product through the automated information system for pharmacovigilance at: https://aisf.dec.gov.ua.
Shelf life
1.5 years.
Do not use after the expiration date specified on the packaging.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 °C in a place inaccessible to children.
Packaging
50 g in bags with an inner packet.
Release category
Without a prescription.
Manufacturer/applicant
Private Joint-Stock Company "Liktрави".
Location of the manufacturer and its address
Ukraine, 10001, Zhytomyr region, Zhytomyr, Kyiv highway, building 21.
- Kraj rejestracji
- Substancja czynna
- Wymaga receptyTak
- Producent
- Te treści mają charakter wyłącznie informacyjny i nie zastępują konsultacji lekarskiej.
- Zamienniki DIACEF 2 GPostać farmaceutyczna: powder, 1000 mg in a vialSubstancja czynna: ceftriaxoneProducent: ТОВ "ЛАБОРАТОРІО ФАРМАСЬЮТІКО Сі.Ті.Wymaga receptyPostać farmaceutyczna: powder, 500mg in a vialSubstancja czynna: ceftriaxoneProducent: ТОВ "ЛАБОРАТОРІО ФАРМАСЬЮТІКО Сі.Ті.Wymaga receptyPostać farmaceutyczna: powder, 1 g, 1 vialSubstancja czynna: ceftriaxoneProducent: Лабораторіо Реіг Жофре, С.А.Wymaga recepty
Odpowiedniki DIACEF 2 G w innych krajach
Leki z tą samą substancją czynną dostępne w innych krajach.
Odpowiednik DIACEF 2 G w Polska
Lekarze online w sprawie DIACEF 2 G
Omów stosowanie DIACEF 2 G, bezpieczeństwo i ocenę zasadności recepty zgodnie z obowiązującymi przepisami.
Często zadawane pytania

Bądź na bieżąco z Oladoctor
Informacje o nowych usługach, aktualizacjach produktu i przydatnych materiałach dla pacjentów.