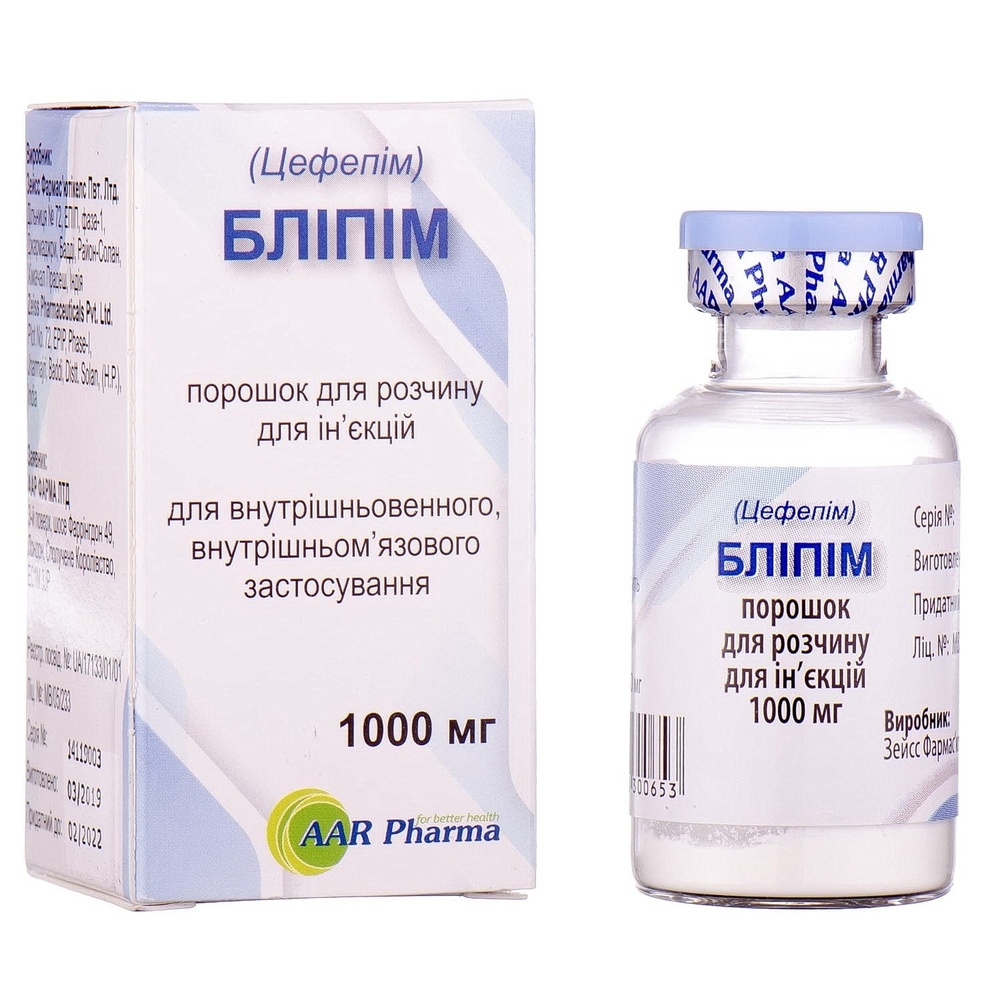
BLIPIM

Jak stosować BLIPIM
Tłumaczenie AI
Ta strona ma charakter informacyjny i nie zastępuje konsultacji lekarskiej. Przed zastosowaniem jakiegokolwiek leku skonsultuj się z lekarzem. Jeśli objawy są poważne, skorzystaj z pilnej pomocy medycznej.
Pokaż oryginałINSTRUCTIONS for medical use of the medicinal product EVKAFILIPTE®(EUCAPHYLIPT)
Composition
The active substance: tincture (1:10) of eucalyptus leaves (Eucalypti folium) (extractant - ethanol 96%); 1 ml of the preparation contains 1 ml of tincture (1:10) of eucalyptus leaves (Eucalypti folium) (extractant - ethanol 96%) in terms of cineole - at least 0.2 mg;
excipient: zinc chloride.
Pharmaceutical form
Tincture.
Main physical and chemical properties
liquid from green to dark green color. During storage, sedimentation is allowed.
Pharmacotherapeutic group
Means used in throat diseases. Antiseptics. ATC code R02A A20.
Pharmacological properties
The tincture contains in its composition tanning substances, ellagic acid, flavonoids, resins, wax, essential oil, which includes various terpene compounds, especially active against gram-positive, gram-negative microorganisms, which have a detrimental effect on fungi and protozoa. Eucalyptus suppresses the growth of golden staphylococcus, Escherichia, tuberculosis mycobacteria, trichomonads, dysentery amoeba. The main component of essential oil is monocyclic terpene cineole. The tincture has an antiseptic, anti-inflammatory and disinfectant effect, which is due to the oxidizing effect of monocyclic terpene cineole and anti-inflammatory influence, associated with almost all physiologically active substances that are part of its composition. The medicinal product also has a moderate sedative (calming) effect.
The preparation exhibits antibacterial activity against staphylococci.
Clinical characteristics
Indications
Inflammatory diseases of the upper respiratory tract, nasopharynx and oral cavity (tracheitis, laryngitis, pharyngitis, gingivitis, stomatitis), skin lesions (including infected wounds), as well as mild forms of neurotic disorders (irritability, emotional lability, sleep disturbances).
Contraindications
Bronchial asthma, bronchospasm. Inflammatory diseases of the gastrointestinal tract and bile ducts. Liver diseases of severe course. Increased individual sensitivity to the medicinal product.
Interaction with other medicinal products and other types of interactions
Not established.
Special instructions
The medicinal product contains ethyl alcohol in an amount of not less than 88.0% (v/v).
Use during pregnancy or breastfeeding
Since the tincture contains ethyl alcohol, it is not recommended to be used internally by women during pregnancy or breastfeeding.
Ability to affect the reaction rate when driving vehicles or other mechanisms
When taking the tincture internally, one should refrain from driving vehicles and working with potentially hazardous mechanisms.
Method of application and doses
The tincture is taken internally, as well as used for rinsing and steam inhalations.
Before use, shake well.
Adults are recommended to take internally, previously dissolving 15-30 drops of the tincture (1 teaspoon) in ¼ cup of boiling water, 3-4 times a day after meals. Children over 12 years old - at the rate of 1 drop per 1 year of life, 3-4 times a day after meals.
For rinsing, dilute 10-15 drops of the tincture in 200 ml of warm boiled water.
Steam inhalations are carried out using inhalers of any type, using the tincture in an undiluted state or after preliminary dilution with water (as for rinsing).
The duration of the medicinal product application depends on the achieved therapeutic effect, which is determined by the doctor.
Children
Since the tincture contains ethyl alcohol, it is not recommended to be prescribed internally to children under 12 years old.
Overdose
Not established.
Adverse reactions
Nausea, vomiting, and diarrhea. With increased sensitivity to the medicinal product, allergic reactions (hives, redness, and skin rash, itching, Quincke's edema) may occur, sometimes - contact dermatitis (in the lip area). In case of pronounced reactions, the use of the preparation should be stopped.
Shelf life
2 years.
Storage conditions
Store in a place inaccessible to children.
Store in the original packaging at a temperature not exceeding 25 °C.
During storage, sedimentation is allowed.
Do not use after the expiration date.
Packaging
100 ml in bottles No. 1 in a pack.
100 ml in jars No. 1 in a pack.
Release category
Without a prescription.
Manufacturer
PRAT "Khimfarmzavod "Chervona Zirka".
Location of the manufacturer and its address
Ukraine, 61010, Kharkiv region, city of Kharkiv, Gorodienkovskaya street, building 1.
- Kraj rejestracji
- Substancja czynna
- Wymaga receptyTak
- Producent
- Te treści mają charakter wyłącznie informacyjny i nie zastępują konsultacji lekarskiej.
- Zamienniki BLIPIMPostać farmaceutyczna: powder, 1000 mg, 1 vialSubstancja czynna: cefepimeProducent: Нектар Лайфсайнсіз Лімітед-Юніт VIWymaga receptyPostać farmaceutyczna: powder, 500 mg, 1 vialSubstancja czynna: cefepimeProducent: Нектар Лайфсайнсіз Лімітед-Юніт VІWymaga receptyPostać farmaceutyczna: powder, 1000 mgSubstancja czynna: cefepimeProducent: Зейсс Фармас’ютікелс Пвт. Лтд.Wymaga recepty
Odpowiedniki BLIPIM w innych krajach
Leki z tą samą substancją czynną dostępne w innych krajach.
Odpowiednik BLIPIM w Polska
Lekarze online w sprawie BLIPIM
Omów stosowanie BLIPIM, bezpieczeństwo i ocenę zasadności recepty zgodnie z obowiązującymi przepisami.
Często zadawane pytania

Bądź na bieżąco z Oladoctor
Informacje o nowych usługach, aktualizacjach produktu i przydatnych materiałach dla pacjentów.