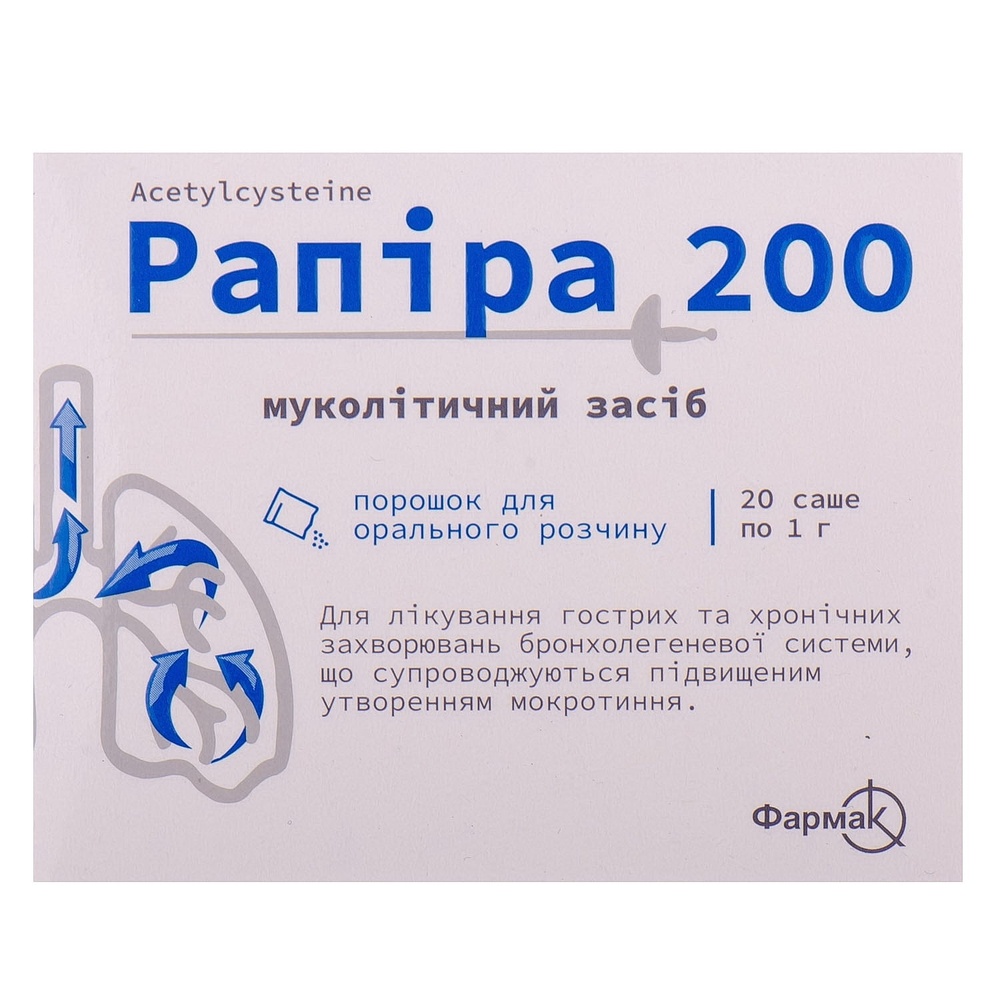
RAPIRA 200

Jak stosować RAPIRA 200
Tłumaczenie AI
Ta strona ma charakter informacyjny i nie zastępuje konsultacji lekarskiej. Przed zastosowaniem jakiegokolwiek leku skonsultuj się z lekarzem. Jeśli objawy są poważne, skorzystaj z pilnej pomocy medycznej.
Pokaż oryginałTreść ulotki
- INSTRUCTIONS for medical use of the medicinal product ISONIAZID-DARNITSA
- Composition
- Pharmaceutical form
- Main physical and chemical properties
- Pharmacotherapeutic group
- Pharmacological properties
- Clinical characteristics
- Interaction with other medicinal products and other types of interactions
- Special instructions
- Use during pregnancy or breastfeeding
- Method of administration and dosage
- Overdose
- Adverse reactions
- Reports of suspected adverse reactions
- Shelf life
- Storage conditions
- Packaging
- Release category
- Manufacturer
- Location of the manufacturer and address of the place of its activity
INSTRUCTIONS for medical use of the medicinal product ISONIAZID-DARNITSA
Composition
active substance: isoniazid; 1 tablet contains 300 mg of isoniazid; excipients: microcrystalline cellulose, methylcellulose, sodium croscarmellose, calcium stearate.
Pharmaceutical form
Tablets.
Main physical and chemical properties
Tablets of round shape with a flat surface, white or white with a creamy tint, with a notch and a facet.
Pharmacotherapeutic group
Anti-tuberculosis drugs. ATC code J04A C01.
Pharmacological properties
Pharmacodynamics
Isoniazid-Darnitsa inhibits DNA-dependent RNA polymerase and inhibits the synthesis of mycolic acids of the cell wall of tuberculosis mycobacteria. The drug has high bacteriostatic activity against tuberculosis mycobacteria, slowing their growth at a concentration of 0.03 mcg/ml. It is especially active against rapidly multiplying microorganisms. It has a weak effect on the causative agents of other infectious diseases.
Pharmacokinetics
Well absorbed from the gastrointestinal tract, easily penetrates the blood-brain barrier. The time to reach the maximum concentration in the blood (Tmax) is 1-4 hours. Binding to plasma proteins is up to 10%. The volume of distribution is 0.56-0.76 L/kg. The tuberculostatic concentration after a single dose is maintained for 6-24 hours. It is widely distributed in the tissues and fluids of the body, including cerebrospinal fluid, pleural effusion, ascitic fluid, skin, lungs, sputum, saliva, and caseous masses. It penetrates through the placenta and is excreted in breast milk.
Clinical characteristics
Indications
- In combination with 3-4 other anti-tuberculosis drugs - for the treatment of active tuberculosis of all forms and localizations;
- as monotherapy - for the treatment of latent tuberculosis infection;
- as monotherapy - for the prevention of tuberculosis in individuals who have been or are in close contact with patients with tuberculosis.
Contraindications
Increased sensitivity to isoniazid or to the excipients of the drug.
Epilepsy and other diseases that are accompanied by a tendency to seizures, severe psychoses, poliomyelitis (including in the anamnesis), toxic hepatitis in the anamnesis due to the use of hydrazide derivatives of isonicotinic acid (ftivazid), pronounced atherosclerosis, acute liver and/or kidney failure.
Interaction with other medicinal products and other types of interactions
Antacid drugs - reduction of isoniazid absorption (the interval between their administration should be at least 1 hour).
Indirect anticoagulants, benzodiazepines, phenytoin, carbamazepine, theophylline, MAO inhibitors - isoniazid potentiates the effects of these drugs (including toxic ones).
Isoniazid may reduce the hepatic metabolism of benzodiazepines (e.g., diazepam, flurazepam, triazolam, midazolam), leading to an increase in their concentration in plasma. Patients should be carefully monitored for signs of benzodiazepine toxicity, and the doses of benzodiazepines should be adjusted accordingly.
Special instructions
As a result of monotherapy with isoniazid, resistant strains of mycobacteria are formed, so it should be used in combination with other anti-tuberculosis drugs. In the case of mixed infection, antibiotics with a broad spectrum of action, fluoroquinolones, and sulfonamides should be prescribed simultaneously with isoniazid.
Use during pregnancy or breastfeeding
During pregnancy, the use of the drug is contraindicated at a dose higher than 10 mg/kg per day. At a dose of up to 10 mg/kg of body weight per day, the use of isoniazid is possible, taking into account the benefit/risk ratio.
Method of administration and dosage
The daily and course dose is determined individually, depending on the course and form of the disease, the degree of inactivation of isoniazid, the effectiveness of therapy, and the tolerance of the drug.
Overdose
Symptoms: in case of overdose, 0.5-3 hours after administration of the drug, disorders of the digestive tract (including liver) may occur - nausea, vomiting, anorexia, metabolic acidosis, acetonuria, hyperglycemia, glucosuria, neurotoxic manifestations (dizziness, fever, headache, visual and hearing disturbances, indistinct speech, weakness, disorientation, visual hallucinations, hyperreflexia, respiratory failure, and depression of the central nervous system, which rapidly progress, stupor, seizures, coma).
Adverse reactions
From the organs of vision: neuritis of the optic nerve, atrophy of the optic nerve.
From the organs of hearing and the vestibular apparatus: hearing loss and tinnitus in patients with terminal stage renal failure.
Reports of suspected adverse reactions
Reports of suspected adverse reactions after registration of the medicinal product are an important procedure.
Shelf life
5 years.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 °C.
Packaging
10 tablets in a contour cell pack; 5 contour cell packs in a pack; 1000, 1500, or 2500 tablets in containers.
Release category
By prescription.
Manufacturer
PrJSC "Pharmaceutical firm "Darnitsa".
Location of the manufacturer and address of the place of its activity
Ukraine, 02093, Kyiv, Borispylska street, 13.
- Kraj rejestracji
- Substancja czynna
- Wymaga receptyNie
- Producent
- Te treści mają charakter wyłącznie informacyjny i nie zastępują konsultacji lekarskiej.
- Zamienniki RAPIRA 200Postać farmaceutyczna: solution, 300mg/3mlSubstancja czynna: acetylcysteineProducent: Мефар Ілач Сан. А.Ш.Wymaga receptyPostać farmaceutyczna: tablets, 200 mgSubstancja czynna: acetylcysteineBez receptyPostać farmaceutyczna: tablets, effervescent tablets 600mgSubstancja czynna: acetylcysteineBez recepty
Odpowiedniki RAPIRA 200 w innych krajach
Leki z tą samą substancją czynną dostępne w innych krajach.
Odpowiednik RAPIRA 200 w Polska
Lekarze online w sprawie RAPIRA 200
Omów stosowanie RAPIRA 200, bezpieczeństwo i ocenę zasadności recepty zgodnie z obowiązującymi przepisami.
Często zadawane pytania

Bądź na bieżąco z Oladoctor
Informacje o nowych usługach, aktualizacjach produktu i przydatnych materiałach dla pacjentów.